Background
Viral hepatitis has always been a major economic burden in the field of public health in China. Although in recent years, with the popularization of hepatitis B vaccine and the inclusion of small molecular antiviral drugs in the medical insurance catalogue, viral hepatitis B and C have been gradually well controlled, the prevalence of hepatitis E is still high, and China is still a high epidemic area of hepatitis E. According to the data of China Center for Disease Control (CDC) in 2019, the seropositive rate of anti-HEV IgG in the general population of China is 27.3%, and the infection rate in coastal areas is higher, and this data may be seriously underestimated.
Hepatitis E virus
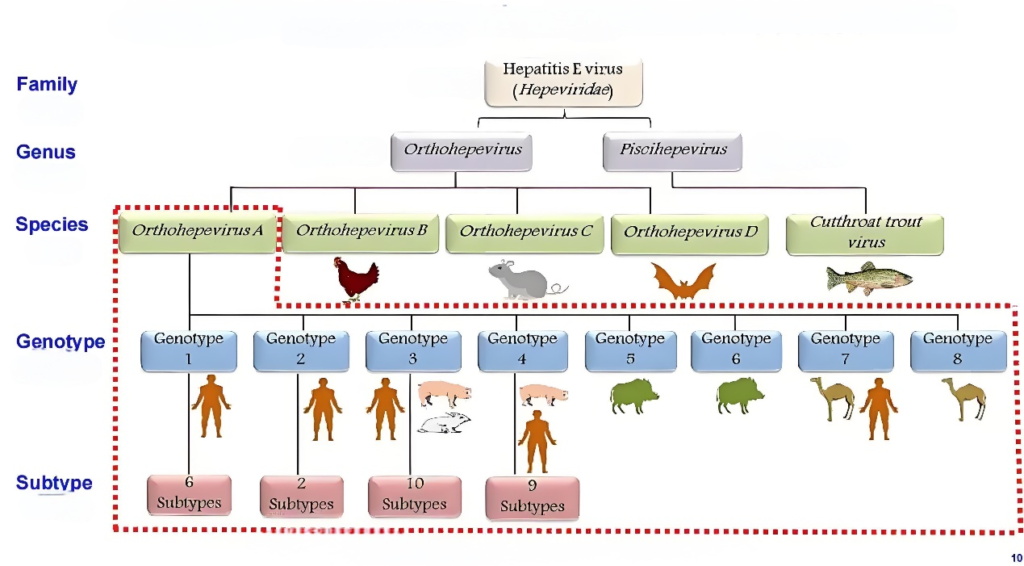
Hepatitis E virus (HEV) is a single-stranded RNA virus with a length of about 7.2 kb. It exists not only in the bile and feces of patients, but also in the blood. The virus is very tenacious at room temperature and can be stored for 28 days and still be infectious, but its infectivity can be eliminated by heating at 70 ℃ for more than 2 minutes. At present, there are 8 genotypes of HEV found in the world, among which the main types of HEV that cause human infection are genotype 1-4, HEV-1 and HEV-2 are easy to cause outbreaks, while HEV-3 is mainly sporadic, and the main types of HEV in China are HEV-1 and 4.
HEV-1 and HEV-2 infect only humans; HEV-3 and HEV-4 can infect humans and a variety of animals, and pigs are the most important hosts. Hepatitis E virus (HEV) type 4 is the most prevalent HEV strain in China.
Epidemiology of hepatitis E
The primary site of HEV replication is the liver, but the virus is also found in other organs, primarily in the small intestine, lymph nodes, and colon. Usually, the virus replicates in the liver and is excreted with feces through the biliary tract, leading to fecal-oral transmission. HEV-1 and HEV-2, which infect only humans and are mainly transmitted by contaminating water supplies with human feces, are highly prevalent in several regions of Asia, Africa, the Middle East, and Mexico. HEV-3 and HEV-4 are zoonotic viruses that can infect humans, pigs and other animal species (including rabbits, wild boars, deer, etc.). There are other genotypes that can be transmitted in camels and marine life. Cross-species transmission usually occurs through direct contact with infected animals and consumption of HEV-contaminated food. HEV can also be transmitted from mother to newborn through vertical transmission; in addition, HEV can also be transmitted through blood transfusion and organ transplantation. All the people who had not been infected with HEV were susceptible to HEV, and most of them were young adults. Some special groups, such as pregnant women, patients with chronic liver diseases and the elderly, are prone to severe liver injury after HEV infection, and even develop into acute or subacute liver failure with high mortality.
Clinical features
The course of acute hepatitis E usually includes several stages. First, there is an incubation period from infection to the onset of symptoms, which is about 3 to 8 weeks. The patient may then go through a brief prodromal phase and then enter a phase in which symptoms are evident or jaundice appears, which may last from a few days to a few weeks. In most published studies, acute hepatitis E usually has a self-limited course, that is, patients can recover spontaneously and generally do not develop chronic hepatitis. The case fatality rate for this hepatitis is highly variable, ranging from 0% to 10%, with an average of about 5%. Of note, no cases of pregnancy-associated acute fulminant hepatitis have been identified in endemic hepatitis E, a condition usually associated with genotype 1 infection. In the United States, large surveys of acute liver failure have found that cases caused by hepatitis E virus (HEV) are very rare, accounting for less than 1% of adult cases.
Endemic hepatitis E has some unique clinical features compared with epidemic or other types of viral hepatitis. In particular, it is more common in specific age and gender groups. In most of the studies, the average age of the patients was over 60, and the number of male patients was at least three times that of female patients. Such sex and age distribution features are not common in other types of viral hepatitis, and these features are not fully explained by known risk factors.
Treatment and prevention
At present, there is no specific antiviral therapy for HEV. Mild cases usually do not require special treatment, while severe cases require symptomatic and supportive treatment. Although drugs such as interferon and ribavirin have certain antiviral effects, their clinical application is limited due to their side effects. Ribavirin may be recommended in some cases, such as in patients after organ transplantation. Recovery of immunity is considered to be a key factor in viral clearance. For patients with liver failure, especially pregnant women, close cooperation of multidisciplinary teams is needed for early diagnosis and intensive care, as well as comprehensive medical treatment, including prevention and treatment of complications, fluid management, maintenance of hemodynamic stability and correction of coagulation dysfunction. In elderly patients, the intervention of artificial liver may help to improve liver function, and liver transplantation may be the key to save lives.
Prevention of hepatitis E is an important measure to avoid the disease, including strengthening personal hygiene, food safety, environmental hygiene and health management of medical institutions, as well as blood processing and screening during the epidemic period, early screening and early treatment.
BoatBio Product Recommendation
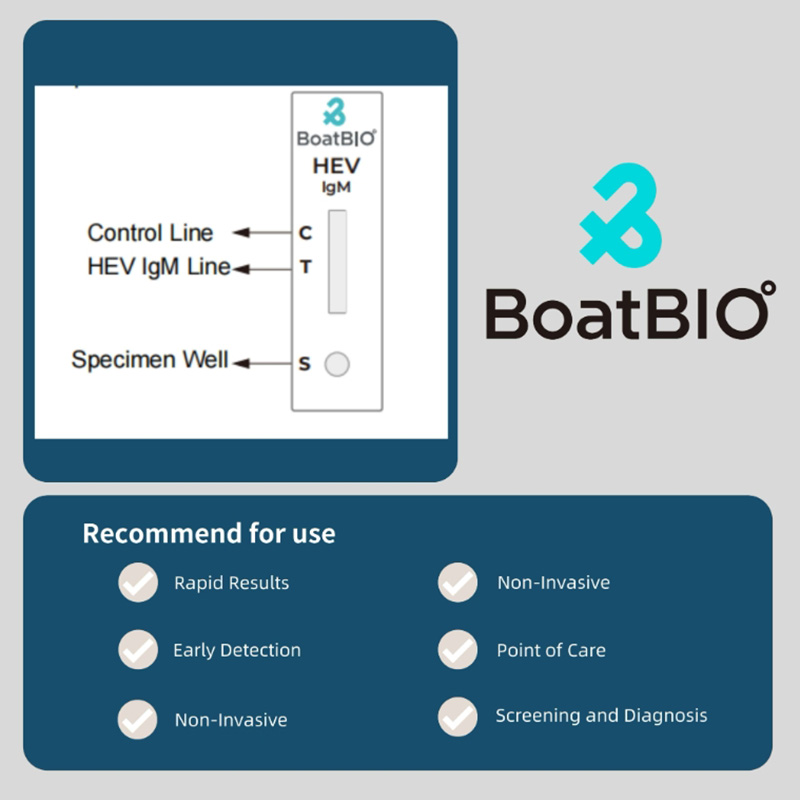
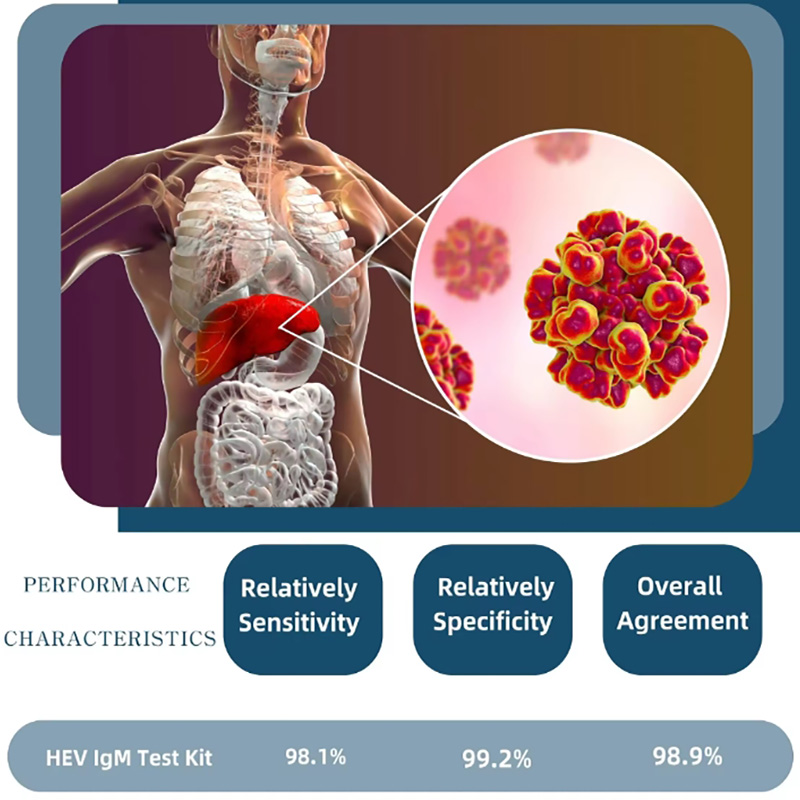
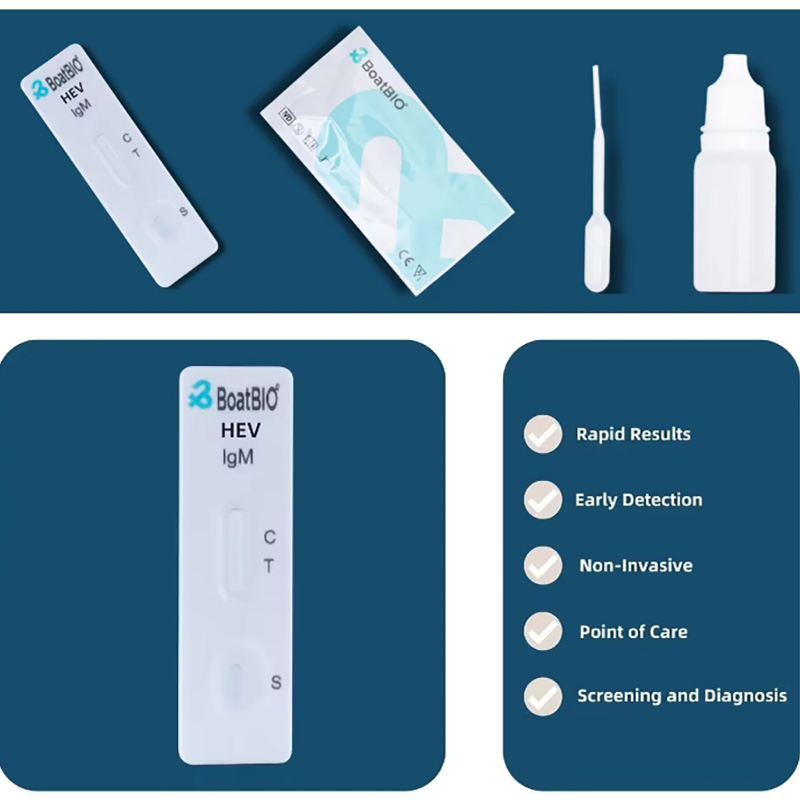
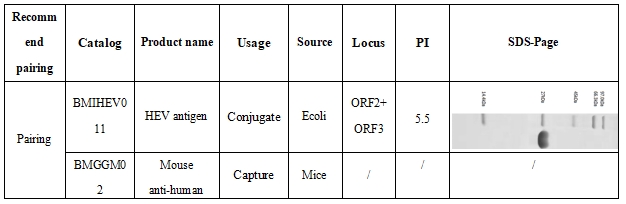
1、HEV-IgM (CMIA) Recommended Matched Material
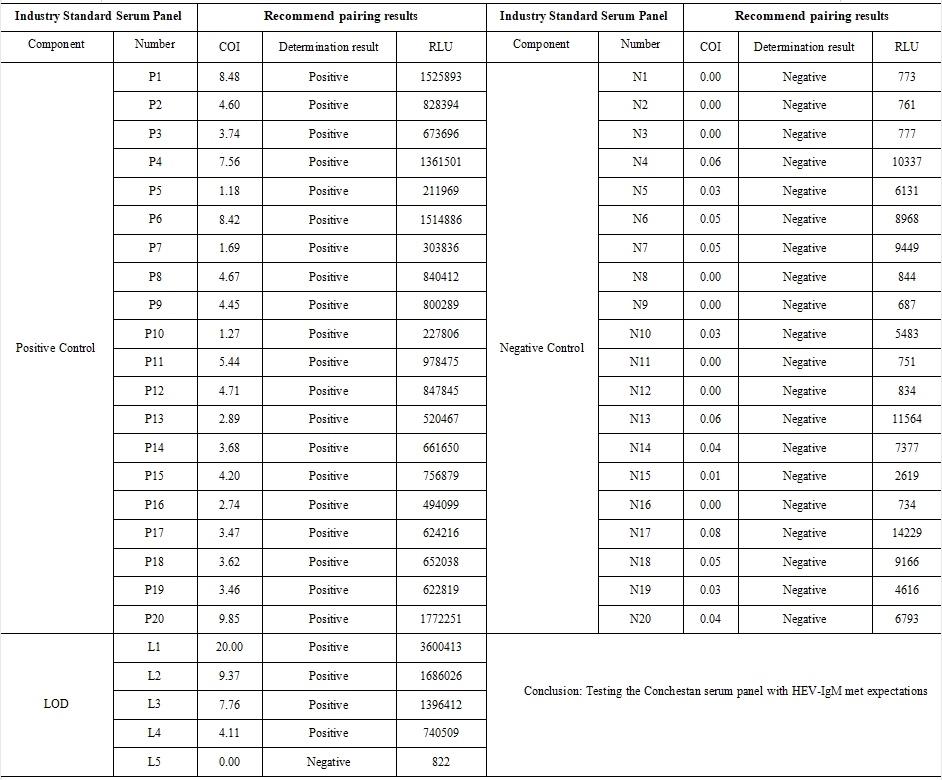
2、 Constash Serum Panel Test Results
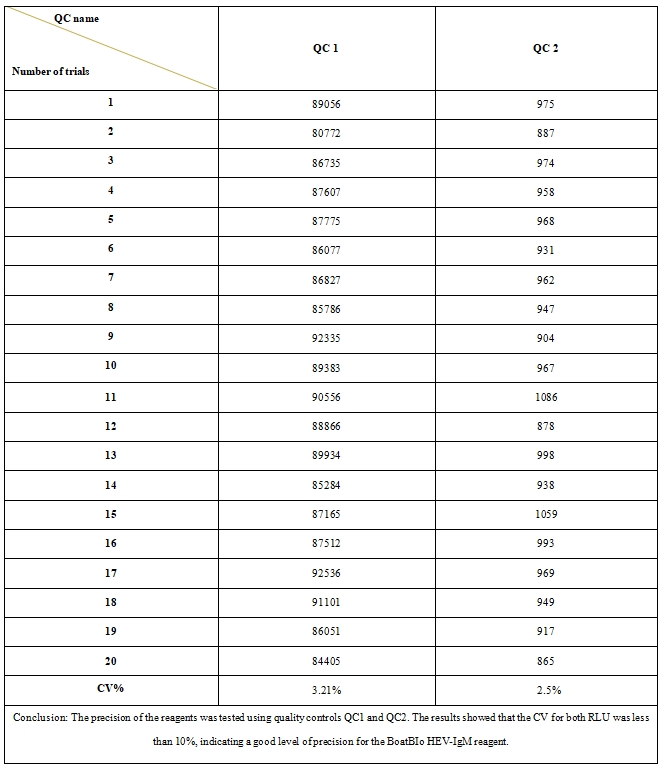
3、Reagent precision
4、Stability test
4.1 Thermal stability
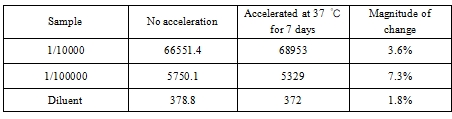
Conclusion: It can be seen that the change range of samples is less than 10%, and the thermal stability of BoatBIo HEV-IgM is good.
4.2 Instrumental stability test results for the reagent
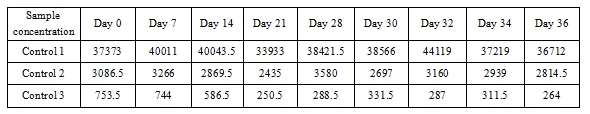
Conclusion: This indicates that the BoatBIo HEV-IgM reagent demonstrates good instrumental test stability.