In recent years, malignant tumor has become one of the major diseases that endanger human health. With the development of economy, the change of lifestyle and the aggravation of environmental pollution, the incidence of cancer is increasing year by year. Because of the insidious onset of cancer, most patients are in the middle and late stages of the disease when they are diagnosed, and miss the best treatment period. Therefore, early screening of cancer has become a key link in the prevention and treatment of cancer. The clinical application of tumor markers provides an important tool for the prevention and treatment of cancer. With the improvement of people’s health awareness, the detection of tumor markers in physical examination has also attracted much attention.
1. What are tumor markers?
Tumor markers, also known as tumor markers, refer to substances that are characteristically present in malignant tumor cells, or produced by tumor cell metabolism, or produced by the host in response to tumor stimulation. Such as glycolytic products, nucleic acid decomposition products, some cytoskeletalcomponents and so on.
It is precisely because tumor markers are the metabolites of cells, in addition to tumor cells, the body is in the case of inflammation or benign diseases, cells may also secrete or produce some metabolites, so the increase of tumor markers does not necessarily mean existence of tumors; Patients with malignant tumors may also have completely normal tumor markers.
2. What is the significance of common tumor markers?
- Alpha-fetoprotein (AFP): AFP disappears from the blood 2 weeks after birth. When liver cells or gonadal embryonic tissues become malignant, AFP in the blood can increase to varying degrees, such as primary hepatocellular carcinoma, ovarian cancer, teratoma, gastric cancer or pancreatic cancer. In addition, viral hepatitis and cirrhosis can also cause a slight increase in AFP.
- Carcinoembryonic antigen (CEA): It is a broad-spectrum tumor marker, which can be expressed in a variety of tumors, such as pancreatic cancer, rectal cancer, breast cancer, lung cancer and gastric cancer. Clinically, it is mainly used for auxiliary diagnosis of malignant tumors, judgment of prognosis and curative effect, and evaluation of tumor recurrence. Generally, CEA concentration may decrease when the condition improves.
- Squamous cell carcinoma antigen (SCC): SCC is a highly specific tumor marker for squamous cell carcinoma. SCC detection is mainly used for the auxiliary diagnosis and prognosis of squamous cell carcinoma of cervix, lung, head and neck and other related sites. The concentration of SCC in serum is related to the differentiation of squamous cell carcinoma.
- Glycoprotein cancer antigen 125 (CA125): normal range: 0 ~ 35 U/mL; CA125 is the preferred marker for ovarian cancer and endometrial cancer, and has a high positive rate in non-small cell lung cancer, and can be increased in patients with pleural effusion and ascites. Mild elevation can be seen in a variety of benign diseases, such as ovarian tumors, uterine fibroids, cervicitis, cirrhosis and hepatitis.
The whey acidic protein (WAP) (HE4) 4-disulfide core domain protein 2 is a secreted glycoprotein first successfully cloned from human epididymal epithelium by Kirchhoff. HE4, a tumor marker for epithelial ovarian cancer, has been approved by the US Food and Drug Administration (FDA) as a marker for monitoring disease progression and recurrence.

3. Misunderstanding of tumor marker screening
1) Is the result of tumor marker “zero” normal?
Because there are “cancer cells” in everyone’s body, while growing more than 10 billion new cells, there will also be 1-20 “cancer cells”. Therefore, almost all the results of tumor markers will not be “zero”, there is a normal range, as long as the value is within the normal range, it is normal. Our immune system can deal with these cancer cells in time, so the average person is less likely to get cancer. Each tumor marker can correspond to one or several tumors, but it is not a one-to-one correspondence. Therefore, when there is a tumor in the body, the corresponding tumor marker may be abnormally elevated, and it is indicated by ↑ on the report sheet.
2) Does the elevation of “swelling mark” lead to cancer?
The elevation of the tumor mark does not indicate cancer, but can only be used for the auxiliary diagnosis of cancer. Some benign diseases and conditions may also cause the elevation of tumor markers.
For example
- Menstrual period, pelvic inflammation and pregnancy may increase CA125;
Biliary tract obstruction, gastritis, inflammatory bowel disease and smoking may increase CEA.
Therefore, it is difficult to diagnose cancer only by the elevation of a tumor, let alone determine which kind of cancer it is, and further examination is needed.
Tumor markers are not the gold standard for the diagnosis of tumors. Therefore, when there is an increase in tumor markers in the physical examination report, it can not be equated with cancer, but it is necessary to arouse some vigilance, dynamically observe the trend of change, and consult specialists when necessary to further clarify the reasons.
Boat-Bio tumor marker
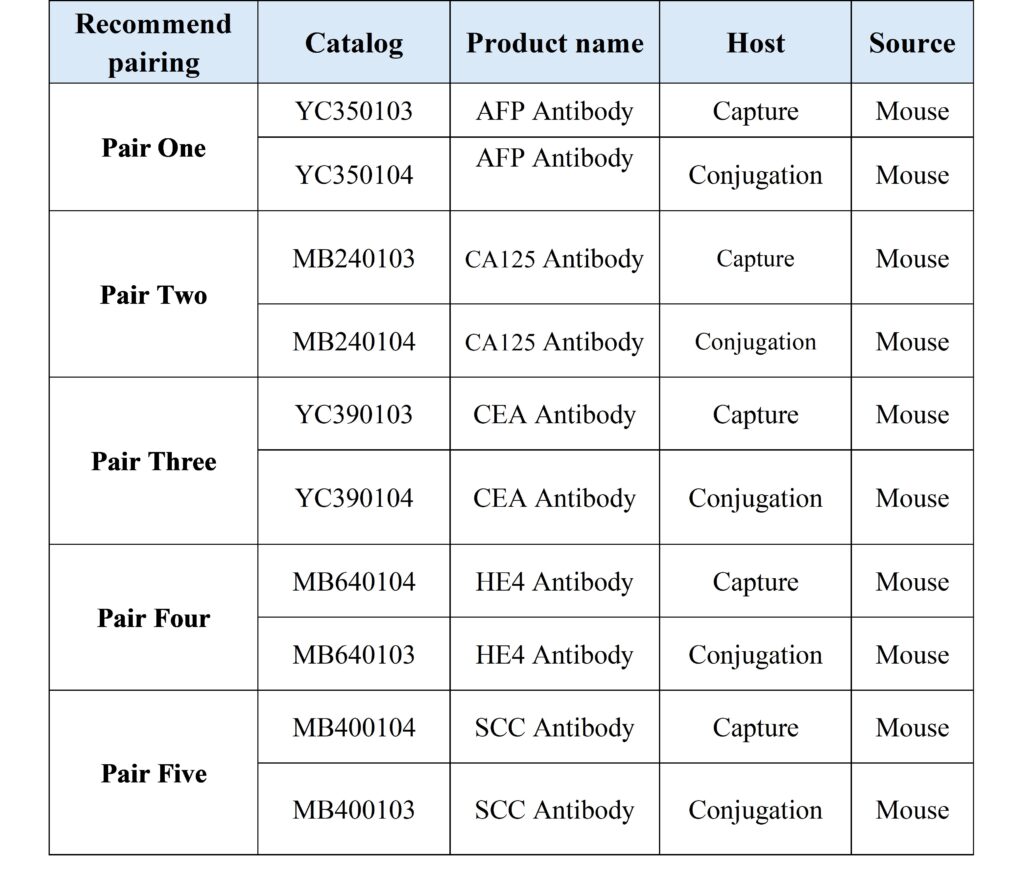
As the upstream core raw material supplier of in vitro diagnosis, Boat-Bio adheres to the goal of “customer-centered, R & D and production of the world’s top products, striving to be a deep partner of IVD enterprises”, and is committed to improving medical efficiency, reducing medical costs and promoting the upgrading of the industry from treatment to prevention through continuous technological innovation and platform exploration. To this end, Boat-Bio independently develops related tumor markers for five conventional tumor markers (CEA, AFP, CA125, HE4 and SCC), with stable product performance and wide application platform.
Product Recommendation
Basic information of raw materials
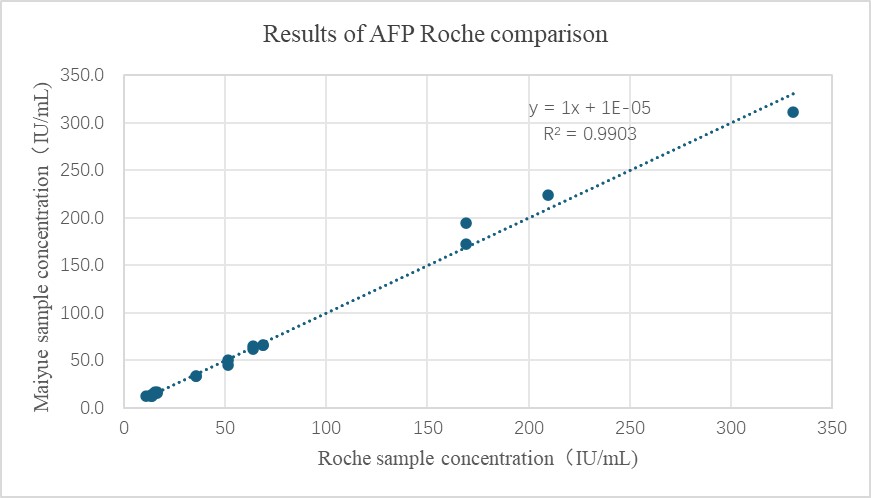
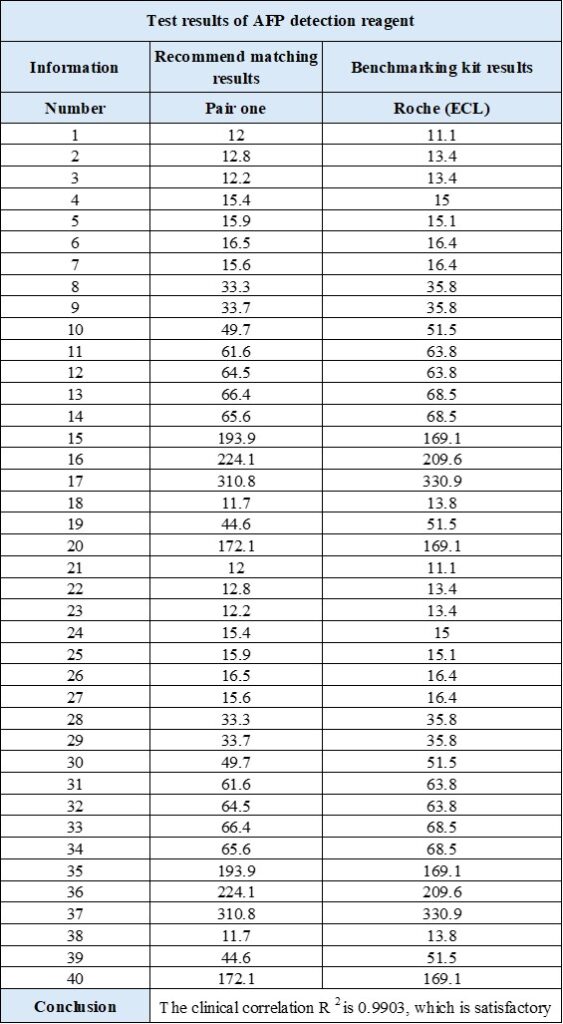
AFP product performance demonstration
- The comparison results of AFP and industry benchmark kits are as follows:
2、 Reagent performance results
2.1 Repeatability
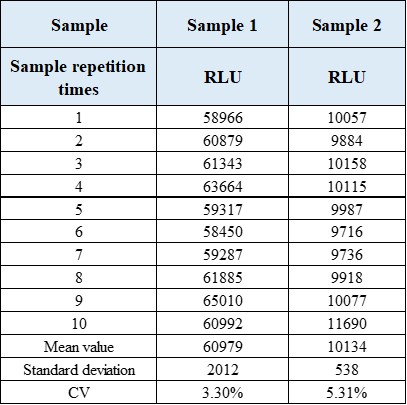
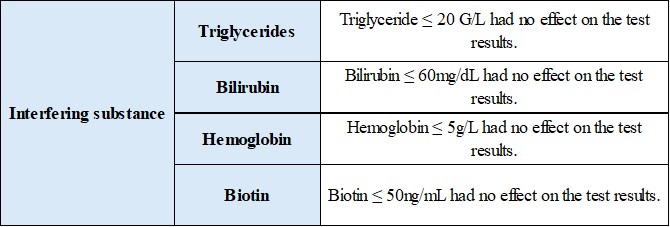
2.2 Anti-interference performance
3、Stability Test Results
3.1 Thermal stability of reagents
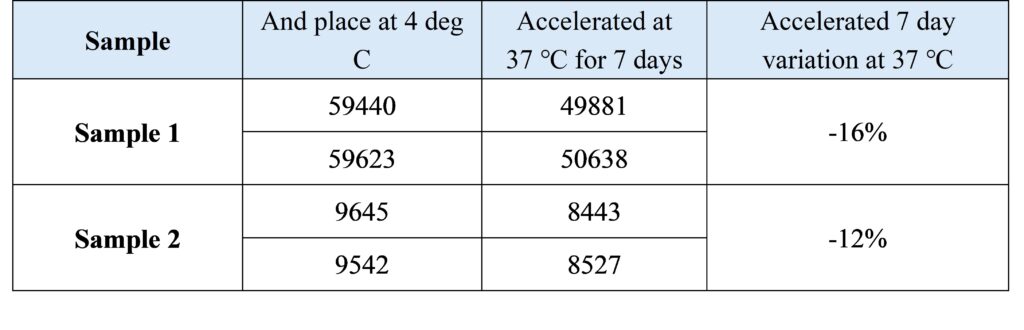
3.2 On-board stabilization of reagents